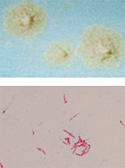
The Mycobacteriology section identifies Mycobacterium species and other aerobic actinomycetes from clinical specimens and from cultures referred on growth media. Specimens are accepted from public and private health care providers.
The Mississippi Public Health Laboratory is the primary laboratory in the state for performing drug susceptibility testing of M. tuberculosis complex.
Test Procedures
Microscopy
Direct microscopic exam with fluorescent or Ziehl-Neelsen stain is performed Monday-Friday. Positive smear results on new patients are considered critical and require same day submitter notification.
Direct detection of Mycobacterium tuberculosis complex using real-time PCR
Real-time PCR for the detection of M. tuberculosis complex is automatically performed on smear-positive respiratory specimens from new patients. Smear-negative specimens are tested by request for patients that meet the tuberculosis case definition for public health surveillance.
Culture and identification of mycobacteria using broth and solid media
Concentrated clinical specimens are cultured using two different media types. The section utilizes the automated Mycobacteria Growth Indicator Tube (MGIT) system and Lowenstein-Jensen slants for the isolation and identification of mycobacteria. Species identification from positive cultures is achieved by MALDI-TOF mass-spectrometry and/or Polymerase Chain Reaction (M. tuberculosis only). Isolates that are confirmed to contain Mycobacterium tuberculosis complex are considered critical and require same day submitter notification. Isolates that are confirmed to contain non-tuberculosis mycobacteria or actinomycetes are reported the same day but do not require submitter notification.
Cultures are incubated for a maximum of six weeks. A report of "no mycobacteria isolated" means that no acid-fast organisms have grown by the end of six weeks. A report of "contaminated" means one of the following: Initial smear positive and no growth on culture, acid-fast bacilli seen on culture but unable to identify, or no acid-fast bacilli seen and culture contaminated.
Antibiotic susceptibility testing
All M. tuberculosis complex isolates are referred to the National PHL Drug Susceptibility Testing Reference Center for first-line DSTs using MGIT with a panel of isoniazid, ethambutol, rifampin, and pyrazinamide. Additional second-line susceptibility testing or molecular detection of drug resistance for select M. tuberculosis complex isolates is also available upon MSDH TB program approval. Drug susceptibility tests on non-tuberculous (NTM) isolates may also be requested and are sent to a reference laboratory at the requestor's expense.
Services Request
Sterile, leak-proof specimen containers and request slips are available upon request. Follow current shipping regulations. If M. haemophilum or M. genavense is suspected, please contact the TB Lab prior to submitting the specimen at (601) 576-7582.
For questions about collection procedures and how to submit specimens please see the Laboratory Services Guide. All requests for clinical testing must be made by a licensed physician or nurse practitioner.

